Electronic Data Capture (EDC) offers numerous advantages, including more precise clinical data that can be easily shared and monitored, enhanced compliance with regulatory standards, and reduced overall study costs.
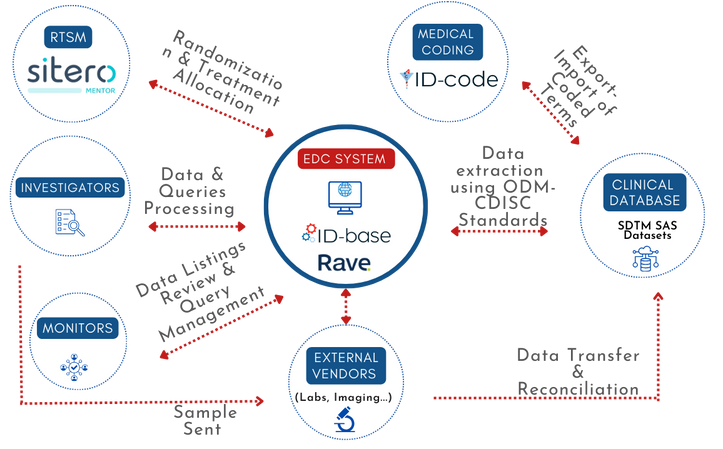
Almustat can configure your eCRF using both ID-base™ (powered by Marvin from EvidentIQ), an EDC system ‘designed BY investigators FOR investigators,’ and the Rave EDC System (powered by Medidata).
Our skilled data managers bring together scientific knowledge, hands-on experience, and technological expertise to ensure the highest value from your clinical data.
At Almustat, we understand that the success of your clinical development program hinges on the quality of your clinical data.
With features designed to reduce costs, enhance site visibility, and boost efficiency, both ID-base™ and Rave offer optimal support for clinical trial processes and enable seamless data flow.
Almustat can configure your eCRF using ID-base™ (powered by Marvin from EvidentIQ), an EDC system ‘designed BY investigators FOR investigators,’ as well as the Rave EDC System (powered by Medidata).
A robust EDC system with a true end-to-end workflow based on CDISC standards, offering real-time electronic data capture to ensure data quality and compliance across all sites.
Key features include:
Getting an accurate diagnosis can be one of the most impactful experiences that you can have.
Copyright © 2024 Almustat.com