All therapeutic areas face specific challenges, from patient populations to endpoints, assessment instruments and other strict study design requirements. If those needs aren’t properly understood, your goals will not be met.
Therefore, it is crucial to partner with an experienced contract research organization that has a solid understanding of the clinical development process and breadth of therapeutic areas expertise.
Our deep therapeutic insight in cancer research and ophthalmology, combined with our expertise in data quality, can significantly impact the success of your clinical trials. With a strong scientific foundation, experienced teams, and in-house therapeutic knowledge, we provide effective support for study design, start-up, and trial management.
Almustat’s extensive expertise is reflected in numerous expert publications in medical and statistical journals, as well as through our active participation in major industry meetings and congresses (available upon request).
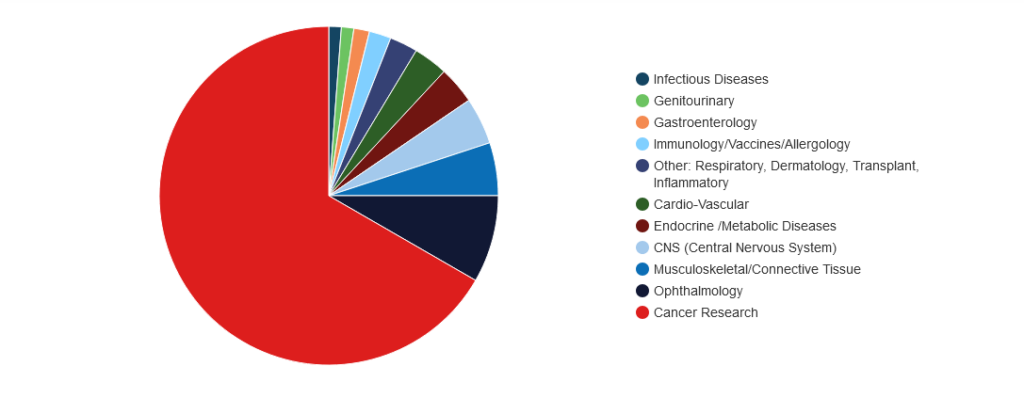
Our broad experience spans over 1,330 clinical trials across diverse therapeutic areas, including drug development, diagnostics, and medical devices. This has enabled us to assist clients in securing 21 FDA/EMA approvals, demonstrating our capacity to handle complex regulatory and trial challenges.
Getting an accurate diagnosis can be one of the most impactful experiences that you can have.
Copyright © 2024 Almustat.com